Page 200 - Personalised medicine of fluoropyrimidines using DPYD pharmacogenetics Carin Lunenburg
P. 200
Chapter 7
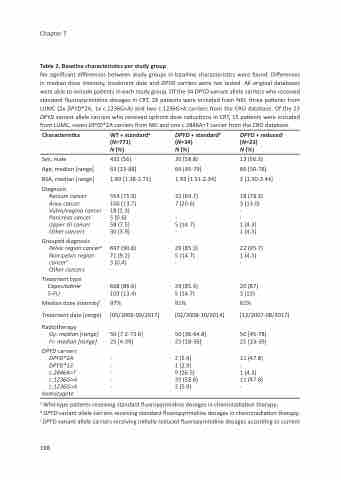
Table 2. Baseline characteristics per study group
No significant differences between study groups in baseline characteristics were found. Differences in median dose intensity, treatment date and DPYD carriers were not tested. All original databases were able to include patients in each study group. Of the 34 DPYD variant allele carriers who received standard fluoropyrimidine dosages in CRT, 29 patients were included from NKI, three patients from LUMC (2x DPYD*2A, 1x c.1236G>A) and two c.1236G>A carriers from the CRO database. Of the 23 DPYD variant allele carriers who received upfront dose reductions in CRT, 15 patients were included from LUMC, seven DPYD*2A carriers from NKI and one c.2846A>T carrier from the CRO database.
Characteristics
Sex, male
Age, median [range]
BSA, median [range]
Diagnosis
Rectum cancer
Anus cancer Vulva/vagina cancer
Pancreas cancer Upper GI cancer Other cancers
Grouped diagnosis
Pelvic region cancerd Non-pelvic region
cancere
Other cancers
Treatment type
Capecitabine 5-FU
Median dose intensityf
Treatment date [range]
Radiotherapy
Gy: median [range] Fr: median [range]
DPYD carriers DPYD*2A DPYD*13
c.2846A>T c.1236G>A c.1236G>A
homozygote
WT + standarda (N=771)
N (%)
432 (56)
63 [23-88]
1.89 [1.38-2.71]
554 (71.9) 106 (13.7) 18 (2.3)
5 (0.6)
58 (7.5) 30 (3.9)
697 (90.8) 71 (9.2)
3 (0.4)
668 (86.6) 103 (13.4)
97% [05/2006-09/2017]
50 [7.2-73.6] 25 [4-39]
- - - - -
DPYD + standardb (N=34)
N (%)
20 (58.8)
64 [45-79]
1.93 [1.51-2.34]
22 (64.7) 7 (20.6) -
-
5 (14.7) -
29 (85.3) 5 (14.7) -
29 (85.3) 5 (14.7)
91% [02/2008-10/2014]
50 [36-64.8] 25 [18-36]
2 (5.9)
1 (2.9)
9 (26.5) 20 (58.8) 2 (5.9)
DPYD + reducedc (N=23)
N (%)
13 (56.5)
66 [50-78]
2 [1.50-2.44]
18 (78.3) 3 (13.0) -
-
1 (4.3) 1 (4.3)
22 (95.7) 1 (4.3)
-
20 (87) 3 (13)
61% [12/2007-08/2017]
50 [45-78] 25 [23-39]
11 (47.8) -
1 (4.3) 11 (47.8) -
a Wild-type patients receiving standard fluoropyrimidine dosages in chemoradiation therapy;
b DPYD variant allele carriers receiving standard fluoropyrimidine dosages in chemoradiation therapy; c DPYD variant allele carriers receiving initially reduced fluoropyrimidine dosages according to current
198